Who Should I Contact About My Project?
Am I Conducting Research?
This decision tool will help you decide whether or not your study is research, as defined by the UK Policy Framework for Health and Social Care Research.
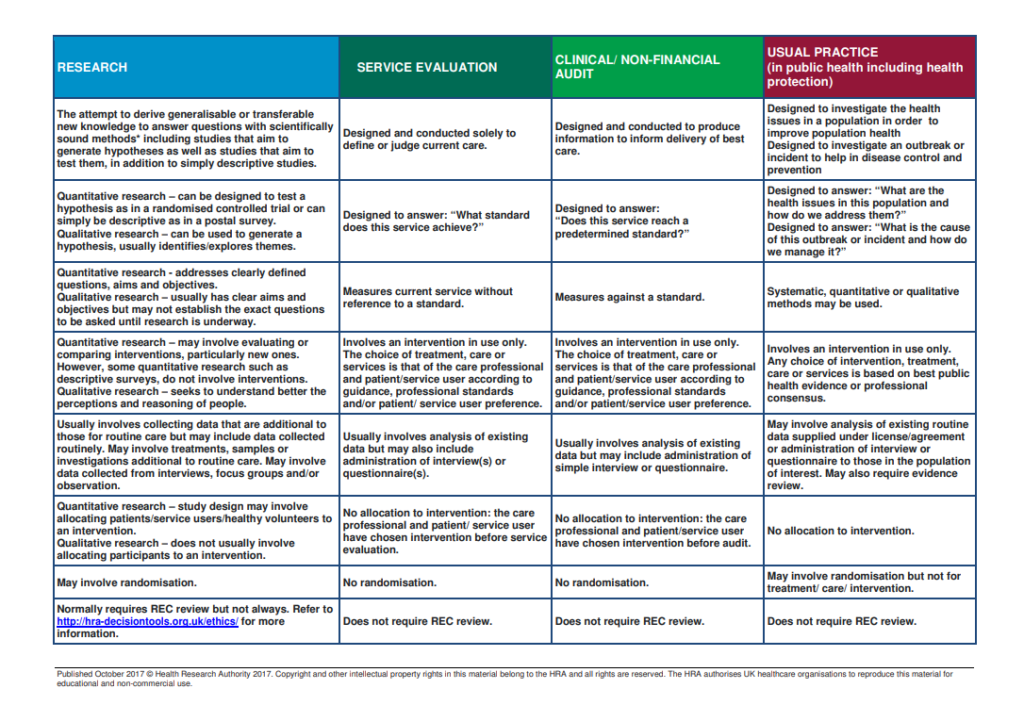
There is also a table listing the differences between research, service evaluation and audit here:
Research
Although we are able to offer some support with research studies, if your project is research, you will need to contact NHS Grampian Research and Development (R&D) for specialised input – for example obtaining permissions, study support and training.
Their site provides information on medical research and research opportunities within NHS Grampian. R&D supports and facilitates all non-commercially funded research for NHS Grampian and manages all research with implications for the NHS on behalf of a consortium comprising NHS Grampian, University of Aberdeen, The Robert Gordon University, Grampian Health Board and the Rowett Research Institute.
You will also need to contact Clinical Research Governance and Quality Assurance.
Their website gives includes detailed information on planning your research study.
This includes how to go about obtaining ethical approval from the National Research Ethics Service – North of Scotland, which is required for most research studies.
Research Ethics Committees are convened to provide the independent advice to participants, researchers, funders, sponsors, employers, care organisations and professionals on the extent to which proposals for research studies comply with recognised ethical standards.
Audit
Although we are able to offer some support with audits, mainly at the planning and analysis or dissemination stages, we suggest you contact the Quality Improvement and Assurance Team for specialised input, including permissions for obtaining data and survey design.
They have resources, offer training, and you can request specific support with your audit.
Contact them at gram.qiat@nhs.scot
Audit projects undertaken within NHS Grampian should be registered with the Clinical Effectiveness Unit. For NHS Grampian staff, the team can provide access to health records for clinical audits, support with data collection and analysis and project management advice/support.
For any project, information provided by the Information Governance Team may be of use.
NHS Grampian staff can obtain information, support and guidance on:
- General Data Protection Regulation (GDPR)
- Data Protection Act 2018
- Freedom of Information (Scotland) Act 2002
- Caldicott and Confidentiality
- National Information Governance Standards
- A Brief Guide to Information Governance
- The Information Governance Strategy
Evaluation
If your project is Evaluation then both the Public Health Research Team and the QIA Team can offer support. Service evaluations undertaken within NHS Grampian should be registered with the QIA Team.
For any project, information provided by the Information Governance Team may be of use.
NHS Grampian staff can obtain information, support and guidance on:
- General Data Protection Regulation (GDPR)
- Data Protection Act 2018
- Freedom of Information (Scotland) Act 2002
- Caldicott and Confidentiality
- National Information Governance Standards
- A Brief Guide to Information Governance
- The Information Governance Strategy
Contact us for more information.